Гонки вакцин 2020
01 марта 2021
Гонки вакцин 2020
- 5515
- 0
- 6
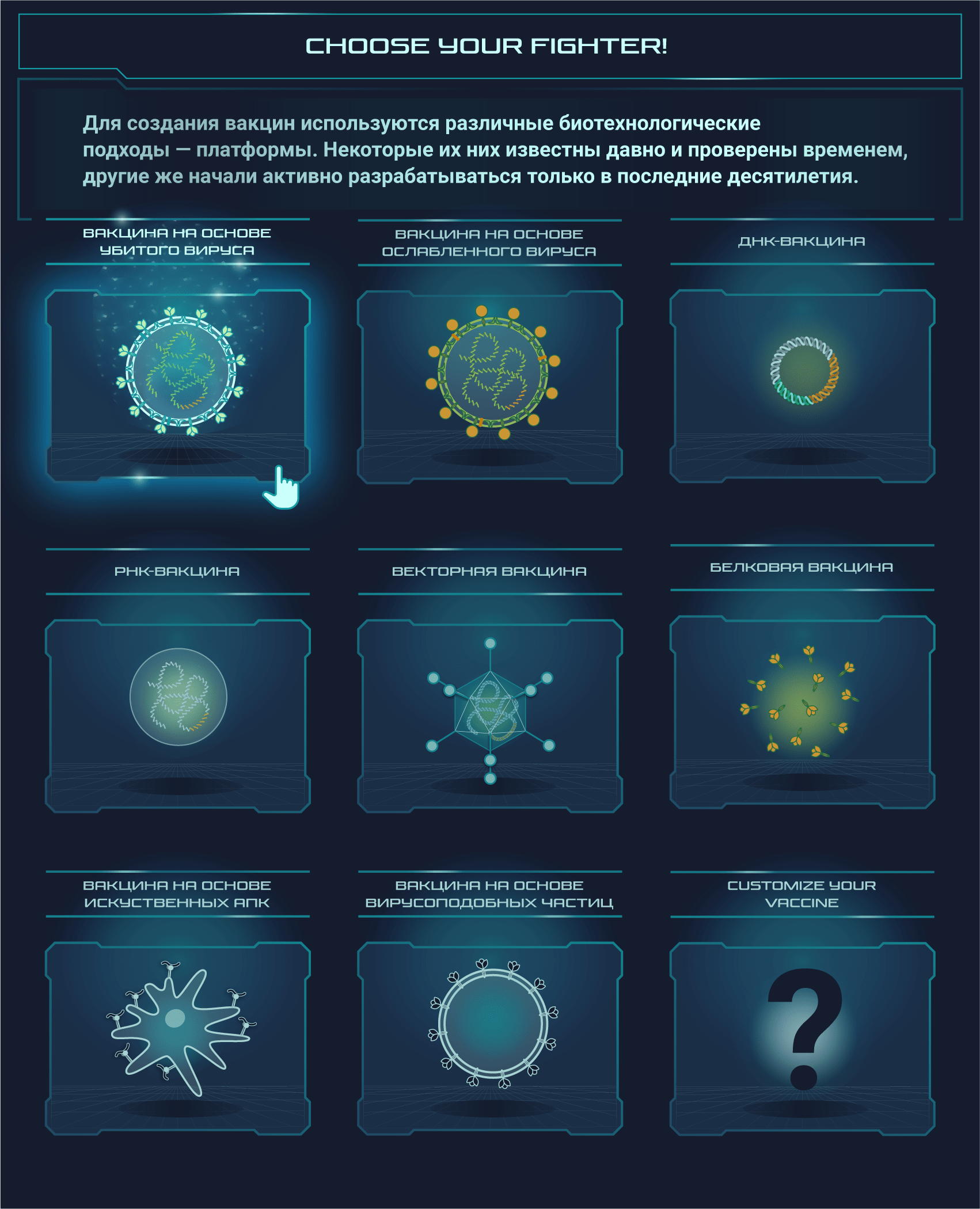
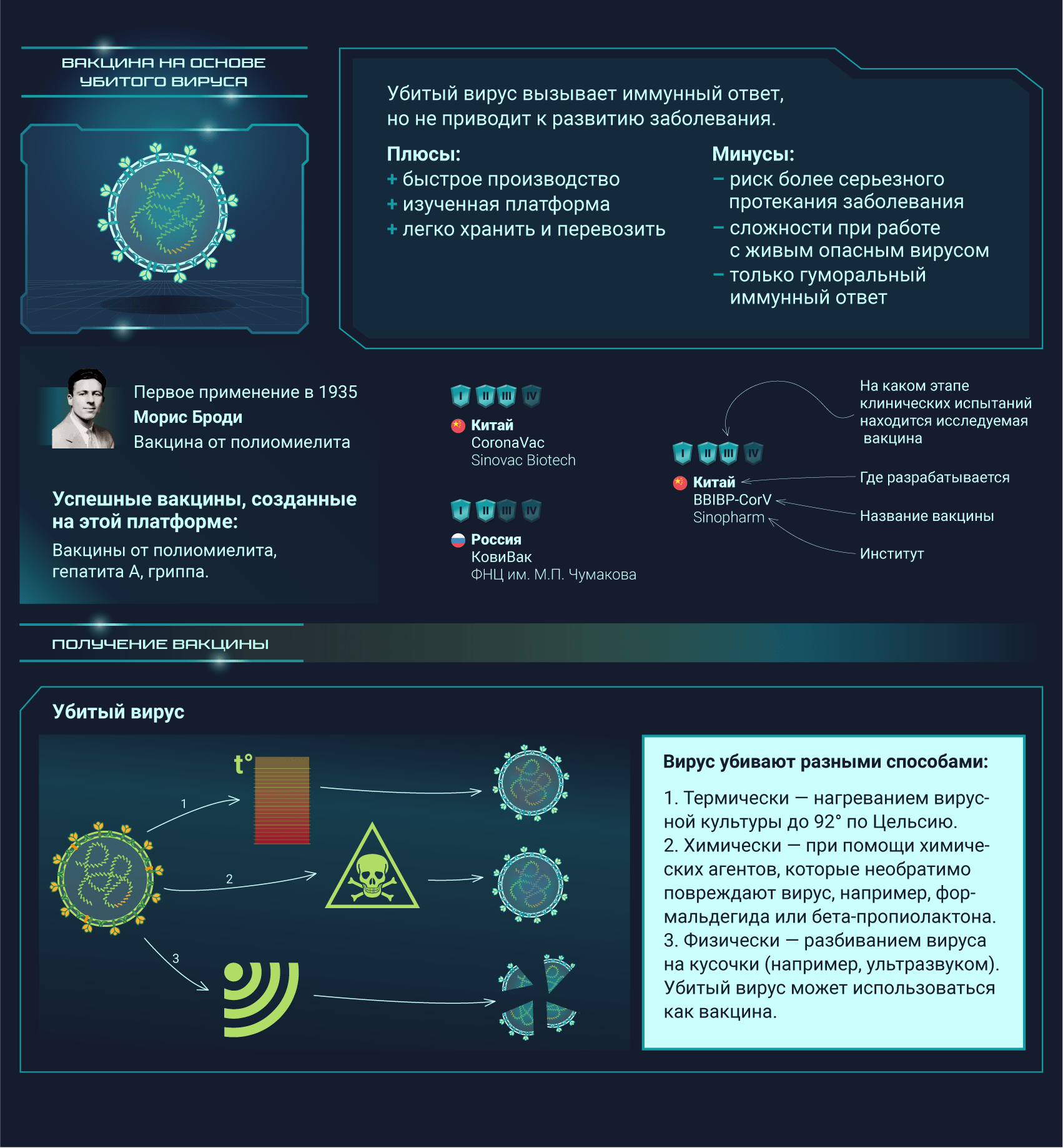
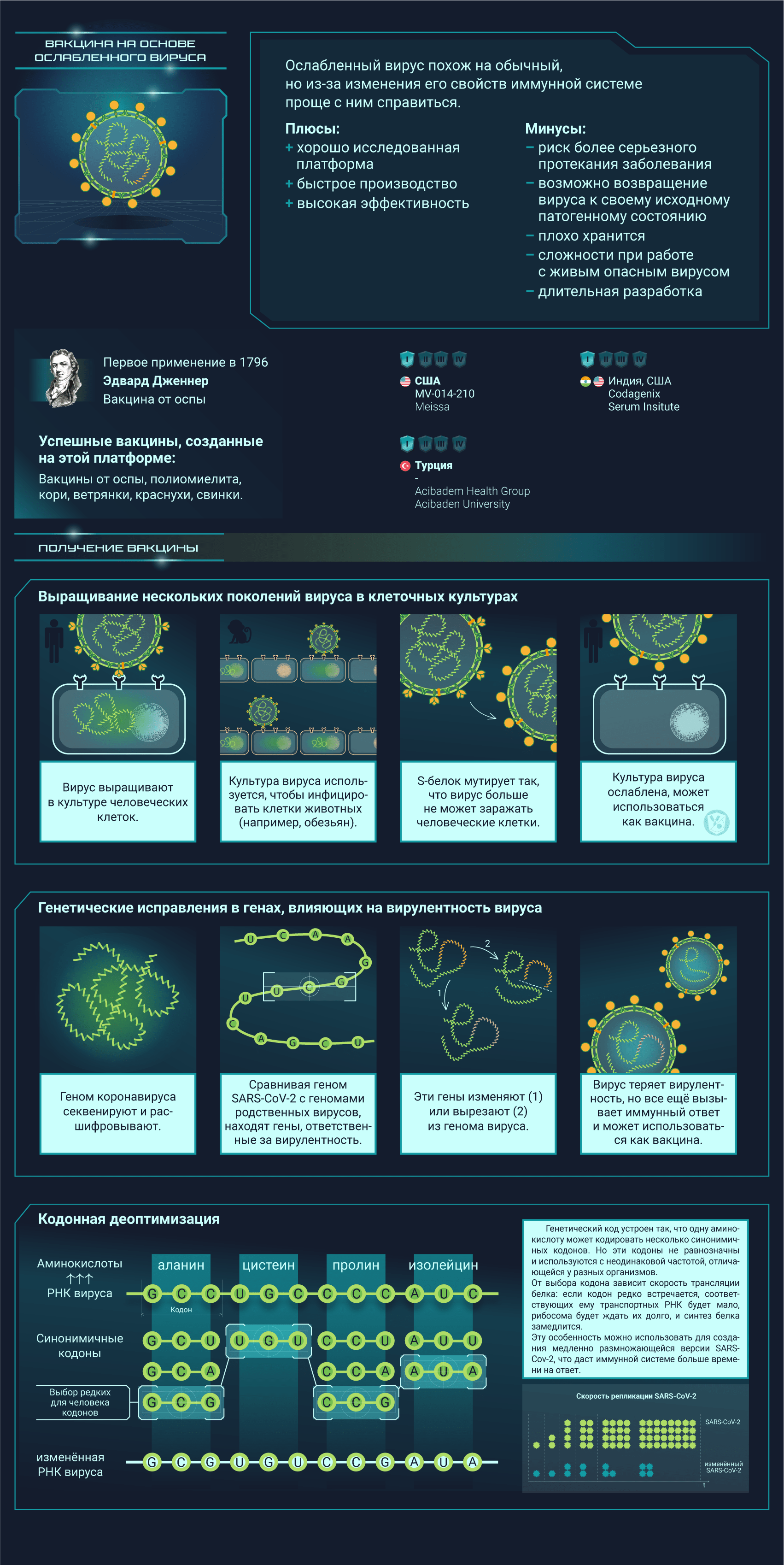
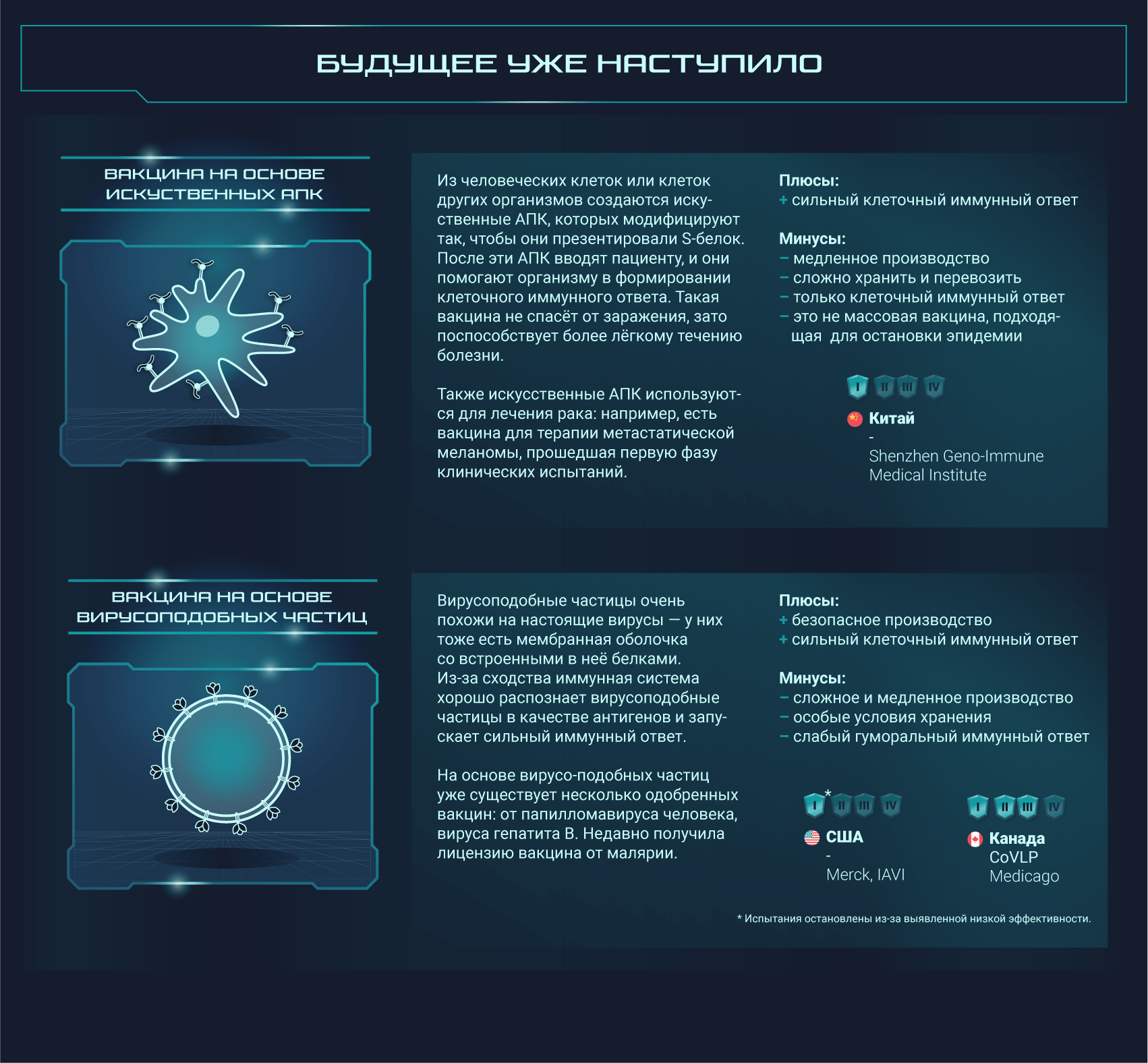
Вакцины от SARS-CoV-2 разрабатываются при помощи самых разных подходов, о которых мы и расскажем
-
Авторы
-
Редакторы
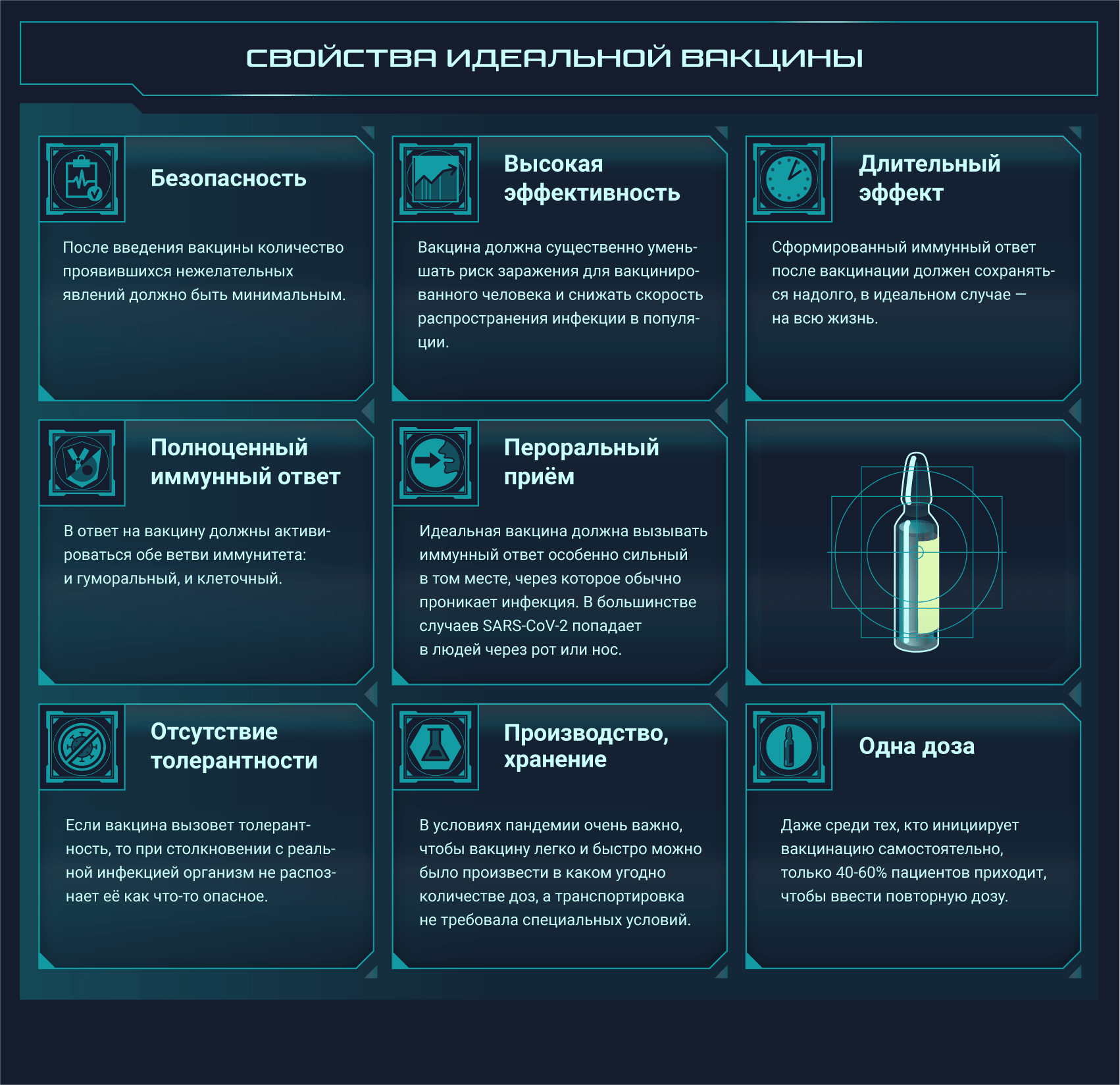
Постер на конкурс «Био/Мол/Текст»: Несмотря на то, что пандемия нового коронавируса застигла человечество врасплох, ученые всего мира быстро начали разработку подходящих вакцин. Наступил значительный перелом в иммунологии: многие из созданных вакцин основаны на новых платформах, поэтому эта эпидемия станет хорошей проверкой для свежих многообещающих подходов. На конец января 2021 года 67 вакцин проходили клинические испытания, а еще как минимум 89 — доклинические. Данный графический конспект поможет разобраться во всем разнообразии существующих платформ, но выбор самой лучшей платформы или тем более выбор самой лучшей вакцины не является его целью.
Конкурс «Био/Мол/Текст»-2020/2021
 Эта работа заняла второе место в номинации «Наглядно о ненаглядном» конкурса «Био/Мол/Текст»-2020/2021.
Эта работа заняла второе место в номинации «Наглядно о ненаглядном» конкурса «Био/Мол/Текст»-2020/2021.

Генеральный партнер конкурса — ежегодная биотехнологическая конференция BiotechClub, организованная международной инновационной биотехнологической компанией BIOCAD.

Спонсор конкурса — компания SkyGen: передовой дистрибьютор продукции для life science на российском рынке.

Спонсор конкурса — компания «Диаэм»: крупнейший поставщик оборудования, реагентов и расходных материалов для биологических исследований и производств.

«Книжный» спонсор конкурса — «Альпина нон-фикшн»
Постер можно скачать в формате pdf по ссылке.


Литература
- Разработка вакцин: чем и как имитировать болезнь?;
- История вакцинации;
- Вакцины против коронавируса: последние новости;
- Wikipedia: Maurice Brodie;
- Wikipedia: Alexander Glenny;
- Руководство по проведению клинических исследований лекарственных средств / Под ред. А.Н. Миронова. М.: «Гриф и К», 2013. — 244 с.;
- Coronavirus global map: tracking the global outbreak. (2021). The New York Times;
- Zimmer C., Corum J., Wee S.-L. (2021). Coronavirus vaccine tracker. The New York Times;
- Consensus document on the epidemiology of severe acute respiratory syndrome (SARS). (2003). WHO;
- A pilot study of a dendritic cell vaccine in HIV-1 infected subjects (PARC002). (2016). Clinical Trials;
- Safety and immunity of Covid-19 aAPC vaccine. (2020). Clinical Trials;
- Abbas A., Lichtman A., Pillai S. Basic immunology: functions and disorders of the immune system (5th Edition). Elsevier, 2015. — 352 p.;
- Yinon M Bar-On, Avi Flamholz, Rob Phillips, Ron Milo. (2020). SARS-CoV-2 (COVID-19) by the numbers. eLife. 9;
- Benson Yee Hin Cheng, Emilio Ortiz-Riaño, Aitor Nogales, Juan Carlos de la Torre, Luis Martínez-Sobrido. (2015). Development of Live-Attenuated Arenavirus Vaccines Based on Codon Deoptimization. J. Virol.. 89, 3523-3533;
- Gerardo Chowell, Fatima Abdirizak, Sunmi Lee, Jonggul Lee, Eunok Jung, et. al.. (2015). Transmission characteristics of MERS and SARS in the healthcare setting: a comparative study. BMC Med. 13;
- Gerardo Chowell, Seth Blumberg, Lone Simonsen, Mark A. Miller, Cécile Viboud. (2014). Synthesizing data and models for the spread of MERS-CoV, 2013: Key role of index cases and hospital transmission. Epidemics. 9, 40-51;
- Guobao Feng, Lu Liu, Wanzhao Cui, Fang Wang. (2020). Electron beam irradiation on novel coronavirus (COVID-19): A Monte–Carlo simulation. Chinese Phys. B. 29, 048703;
- H. J. Hearn, W. T. Soper, W. S. Miller. (1965). Loss in Virulence of Yellow Fever Virus Serially Passed in HeLa Cells. Experimental Biology and Medicine. 119, 319-322;
- Natalia G. Herrera, Nicholas C. Morano, Alev Celikgil, George I. Georgiev, Ryan J. Malonis, et. al. Characterization of the SARS-CoV-2 S Protein: Biophysical, Biochemical, Structural, and Antigenic Analysis — Cold Spring Harbor Laboratory;
- Dominika Hobernik, Matthias Bros. (2018). DNA Vaccines—How Far From Clinical Use?. IJMS. 19, 3605;
- Susanne H Hodgson, Kushal Mansatta, Garry Mallett, Victoria Harris, Katherine R W Emary, Andrew J Pollard. (2021). What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. The Lancet Infectious Diseases. 21, e26-e35;
- Yuan Huang, Chan Yang, Xin-feng Xu, Wei Xu, Shu-wen Liu. (2020). Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 41, 1141-1149;
- Elmira T. Isakbaeva, Nino Khetsuriani, R. Suzanne Beard, Angela Peck, Dean Erdman, et. al.. (2004). SARS-associated Coronavirus Transmission, United States. Emerg. Infect. Dis.. 10, 225-231;
- D. A. Jackson, R. H. Symons, P. Berg. (1972). Biochemical Method for Inserting New Genetic Information into DNA of Simian Virus 40: Circular SV40 DNA Molecules Containing Lambda Phage Genes and the Galactose Operon of Escherichia coli. Proceedings of the National Academy of Sciences. 69, 2904-2909;
- Mangalakumari Jeyanathan, Sam Afkhami, Fiona Smaill, Matthew S. Miller, Brian D. Lichty, Zhou Xing. (2020). Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 20, 615-632;
- Young Chan Kim, Barbara Dema, Arturo Reyes-Sandoval. (2020). COVID-19 vaccines: breaking record times to first-in-human trials. npj Vaccines. 5;
- Yizhar Lavner, Daniel Kotlar. (2005). Codon bias as a factor in regulating expression via translation rate in the human genome. Gene. 345, 127-138;
- H. Lei, Y. Li, S. Xiao, C.-H. Lin, S. L. Norris, et. al.. (2018). Routes of transmission of influenza A H1N1, SARS CoV, and norovirus in air cabin: Comparative analyses. Indoor Air. 28, 394-403;
- Char Leung. (2020). The difference in the incubation period of 2019 novel coronavirus (SARS-CoV-2) infection between travelers to Hubei and nontravelers: The need for a longer quarantine period. Infect. Control Hosp. Epidemiol.. 41, 594-596;
- M. A. Liu. (2003). DNA vaccines: a review. J Intern Med. 253, 402-410;
- Andreas Mackensen, Norbert Meidenbauer, Sandra Vogl, Monika Laumer, Jana Berger, Reinhard Andreesen. (2006). Phase I Study of Adoptive T-Cell Therapy Using Antigen-Specific CD8+ T Cells for the Treatment of Patients With Metastatic Melanoma. JCO. 24, 5060-5069;
- Jamie FS Mann, Reinaldo Acevedo, Judith del Campo, Oliver Pérez, Valerie A Ferro. (2009). Delivery systems: a vaccine strategy for overcoming mucosal tolerance?. Expert Review of Vaccines. 8, 103-112;
- Frédéric Martinon, Sivadasan Krishnan, Gerlinde Lenzen, Rémy Magné, Elisabeth Gomard, et. al.. (1993). Induction of virus-specific cytotoxic T lymphocytesin vivo by liposome-entrapped mRNA. Eur. J. Immunol.. 23, 1719-1722;
- Mona O. Mohsen, Lisha Zha, Gustavo Cabral-Miranda, Martin F. Bachmann. (2017). Major findings and recent advances in virus–like particle (VLP)-based vaccines. Seminars in Immunology. 34, 123-132;
- Murphy K. and Weaver C. Janeway’s immunobiology (9th Edition). W.W. Norton & Company, 2016. — 924 p.;
- Ji-Eun Park, Soyoung Jung, Aeran Kim, Ji-Eun Park. (2018). MERS transmission and risk factors: a systematic review. BMC Public Health. 18;
- Linda J. Saif. (2020). VACCINES FOR COVID-19: PERSPECTIVES, PROSPECTS, AND CHALLENGES BASED ON CANDIDATE SARS, MERS, AND ANIMAL CORONAVIRUS VACCINES. EMJ;
- Alan Sariol, Stanley Perlman. (2020). Lessons for COVID-19 Immunity from Other Coronavirus Infections. Immunity. 53, 248-263;
- Thomas Schlake, Andreas Thess, Mariola Fotin-Mleczek, Karl-Josef Kallen. (2012). Developing mRNA-vaccine technologies. RNA Biology. 9, 1319-1330;
- Eunha Shim, Alison P. Galvani. (2012). Distinguishing vaccine efficacy and effectiveness. Vaccine. 30, 6700-6705;
- Melissa S. Stockwell, Annika M. Hofstetter, Nathalie DuRivage, Angela Barrett, Nadira Fernandez, et. al.. (2015). Text Message Reminders for Second Dose of Influenza Vaccine: A Randomized Controlled Trial. Pediatrics. 135, e83-e91;
- J. S. Tregoning, E. S. Brown, H. M. Cheeseman, K. E. Flight, S. L. Higham, et. al.. (2020). Vaccines for COVID‐19. Clin. Exp. Immunol.. 202, 162-192;
- De-chu Tang, Michael DeVit, Stephen A. Johnston. (1992). Genetic immunization is a simple method for eliciting an immune response. Nature. 356, 152-154;
- J. S. Tregoning, E. S. Brown, H. M. Cheeseman, K. E. Flight, S. L. Higham, et. al.. (2020). Vaccines for COVID‐19. Clin. Exp. Immunol.. 202, 162-192;
- A. R. Tuite, A. L. Greer, M. Whelan, A.-L. Winter, B. Lee, et. al.. (2010). Estimated epidemiologic parameters and morbidity associated with pandemic H1N1 influenza. Canadian Medical Association Journal. 182, 131-136;
- Brian J Ward, Philipe Gobeil, Annie Séguin, Judith Atkins, Iohann Boulay, et. al. Phase 1 trial of a Candidate Recombinant Virus-Like Particle Vaccine for Covid-19 Disease Produced in Plants — Cold Spring Harbor Laboratory;
- Gizachew Tadesse Wassie, Abebaw Gedef Azene, Getasew Mulat Bantie, Getenet Dessie, Abiba Mihret Aragaw. (2020). Incubation Period of Severe Acute Respiratory Syndrome Novel Coronavirus 2 that Causes Coronavirus Disease 2019: A Systematic Review and Meta-Analysis. Current Therapeutic Research. 93, 100607;
- Benjamin Weide, Steve Pascolo, Birgit Scheel, Evelyna Derhovanessian, Annette Pflugfelder, et. al.. (2009). Direct Injection of Protamine-protected mRNA: Results of a Phase 1/2 Vaccination Trial in Metastatic Melanoma Patients. Journal of Immunotherapy. 32, 498-507.